Accelerating Action: Empowering Women in Regulatory Affairs
Published Mar 06, 2025
Published 26th February 2025
Growth in the development of orphan drugs continues rapidly, driven by a rising patient population and investments in novel technologies generating advanced therapies. Orphan drugs now account for around 15% of sales in the pharmaceutical sector and are among the most profitable pharmaceuticals in the world. Many of the higher-priced treatments target cancer, but other disease areas, particularly neurology, are also following this path. The oncology sector holds the largest share of the orphan drug market, with Revlimid and Darzalex, (multiple myeloma treatments), being amongst the most profitable drugs in 2024.
Maintaining orphan drug designation (ODD) during a Marketing Authorisation Application (MAA) benefits patients and drug developers. It may enable faster price negotiation and quicker access for patients with the orphan condition. The criteria for maintaining orphan status at MAA is the same as at initial designation – “if satisfactory methods exist, the medicine must be of significant benefit to those affected by the condition” as set in the Orphan Regulation (EC) No 141/2000 – but the level of evidence for supporting significant benefit (SB) is higher and requires significant clinical data.
As successful research and investment grows in a disease or condition, the number of products available increases over time. Advanced therapies may raise the bar for efficacy but may also present a challenge for widespread access due to eligibility and cost. For new products, the complexity of providing comparative and compelling data vs all relevant treatments increases also. By looking at examples in the multiple myeloma setting, this article summarises top tips on how to optimise success in maintaining orphan status by learning from a crowded market.
Treatment evolution has greatly extended the survival of multiple myeloma patients, at least doubling the 5-year survival average over the last 30 years. It is a rare disease per EU criteria, with a prevalence of less than 5/10,000. However, an EMA report forecasts that this threshold will likely be crossed by 2025.
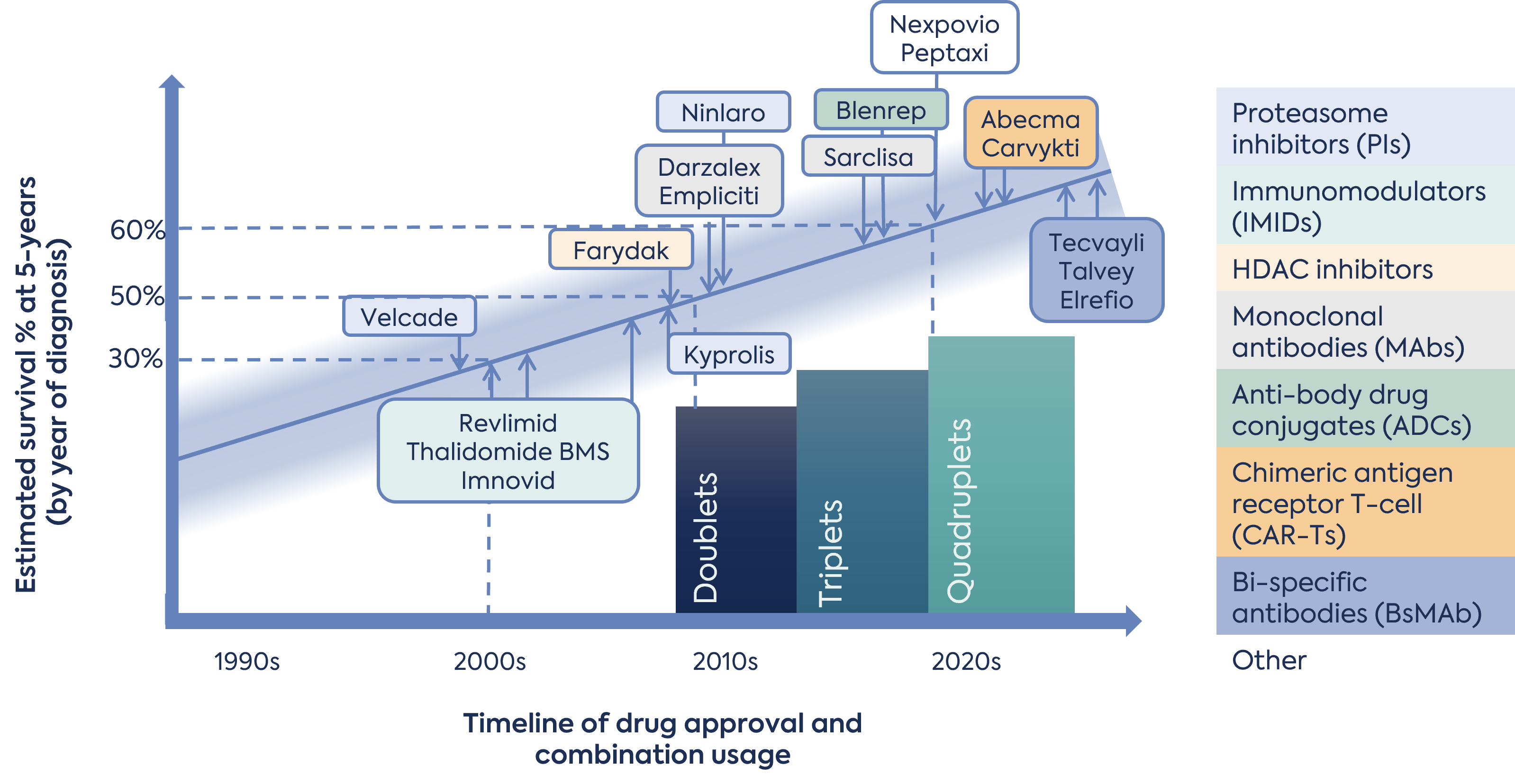
The number and modalities of available treatments has increased, and in particular, within the last 10 years. Since 2015, EMA has authorised 14 new products with novel mechanisms or active substances. Nine of these were authorised since 2020. Modalities authorised include small molecule inhibitors, monoclonal antibodies, antibody-drug conjugates (ADC), bispecific antibodies and chimeric antigen receptors (CAR)-T cell therapies (Figure 1). Of these products, regulators initially authorised 9 under a conditional marketing authorisation (CMA), designated 6 with PRIME, and granted 3 under accelerated assessment (AA). This highlights the unmet need that remains for this disease, despite the number of approved treatments.
New treatment options have increased the development and understanding of effective treatment combinations for patient sub-populations. The EU ESMO-EHA clinical guidelines describe various doublet, triplet and quadruplet combinations depending on the patient attributes and prior treatments.
The applicants of each one of the 14 products approved since 2015 requested to maintain orphan status at initial MAA, of which, only 8 were successful. At least 5 of these applicants sought protocol assistance (PA) on how to demonstrate significant benefit at the time of MAA. Despite this, not all led to a positive opinion to maintain orphan status in all cases (Table 1).
| Product (1) | PRIME | AA | CMA | PA on SB | OD maintained |
|---|---|---|---|---|---|
| Farydak | - | No | No | * | Yes |
| Kyprolis | - | Yes | No | * | Yes |
| Empliciti | - | Yes | No | * | Withdrawn |
| Darzalex | - | Yes | Yes | * | Yes |
| Ninlaro | - | No | Yes | * | Yes |
| Sarclisa | No | No | No | No | Withdrawn |
| Blenrep | Yes | No | Yes | Yes | Yes |
| Nexpovio | No | No | Yes | * | Withdrawn |
| Abecma | Yes | No | Yes | * | Yes |
| Carvykti | Yes | No | Yes | Yes | Yes |
| Pepaxti | No | No | No | No | Withdrawn |
| Tecvayli | Yes | No | Yes | Yes | Withdrawn |
| Talvey | Yes | No | Yes | Yes | Yes |
| Elrexfio | Yes | No | Yes | Yes | Withdrawn |
(1) Ordered oldest to latest authorised. – PRIME designation introduced in 2016, * EPAR and other sources unable to confirm Y/N.
With an evolving treatment landscape, the first challenge when tackling significant benefit can be identifying relevant products for which comparative data for significant benefit should be provided. A combination of the orphan condition and the MAA indication should be used. The EU guidance and orphan maintenance report template requires applicants to list authorised treatments in the condition and discuss the EU consensus clinical guidelines. The condition does not include subsets of patients, i.e. the condition is ‘Treatment of multiple myeloma’. Therefore, listing all authorised treatments in the defined orphan condition as relevant to significant benefit may be redundant. This is because treatments may be directed by disease stage, severity, prior treatments, and patient characteristics beyond the approved indication.
In the cases explored for multiple myeloma, the earliest maintenance report assessment (Farydak) discussed 6 classes of treatments recognised in the clinical guideline at that time and provided direct survival data from the phase 3, placebo controlled, registrational trial in subjects who had previously received the latest most efficacious treatments (proteasome inhibitors and immunomodulatory drugs). Likewise, 2 other products authorised around this time (2015-2016) provided direct data from Phase 3, controlled trials along with some indirect comparisons for the 3-5 products already authorised and in-line with the indication.
As time progressed the overall number of treatments in the condition increased to more than 27 in all. However, narrowing this list based on clinical guidelines and proposed ‘line of therapy’ defined in the indication for the MAA is appropriate to select the products to compare and demonstrate significant benefit. In a condition with a fast pace of expansion the likely relevant existing treatments can change during a product’s development. Thus regular re-evaluation of the likely list and proactive data gathering for products not yet authorised may be necessary.
To demonstrate significant benefit, the Orphan Regulation ((EC) No 141/2000) and complimentary Commission notice (2016/C 424/03) outline two grounds:
OR
The ideal data to demonstrate significant benefit would be improved efficacy from a controlled trial with existing therapy as an active comparator. But with few patients (small studies) and a long list of authorised therapies, how can we successfully show benefit?
In the last 5 years, nine novel medicines were evaluated for an orphan MAA in relapsed/refractory multiple myeloma. Various data approaches were used, including bridging to other trials, prospective indirect comparisons, and side-by-side assessment of pivotal trial data. In addition, incorporation of real-world data to provides evidence and context for outcomes over time. (Table 2).
| Product (1) | Number of products relevant for SB comparison (2) | Type of data included (2) | OD maintained at MAA? |
|---|---|---|---|
| Sarclisa | 6 | • Ph3 data vs backbone treatment | Withdrawn |
| Blenrep | 1 | • Subjects response following prior treatment • Bridge to observational study • MAIC* analysis • RWE on responses in sub-group | Yes |
| Nexpovio | 1 | • Ph2 single arm data | Withdrawn |
| Abecma | 2 | • Side-by-side study responses • Quality of life (QOL) study data | Yes |
| Carvykti | 3 | • MAIC analysis | Yes |
| Pepaxti | 5 | • Population comparison to available therapy | Withdrawn |
| Tecvayli | 3 | • Subjects response following prior treatment • MAIC analysis | Withdrawn |
| Talvey | 5 | • Subjects response following prior treatment • Observational data • Side-by-side study responses | Yes |
| Elrexfio | 7 | • Subjects response following prior treatment | Withdrawn |
(1) Ordered oldest to latest authorised, (2) Based on published maintenance report, * Matching-adjusted indirect comparison – MAIC.
New modalities, e.g. advanced therapies or bi-specific monoclonal antibodies, can add to the challenge of generating comparative efficacy data. This is because the response rate to treatment is high with targeted immune therapies. Supporting grounds based on safety and/or MCPC have not been successful in the reviewed multiple myeloma cases. In selected examples where a MCPC was presented, the COMP noted that from a clinical perspective, the potential for new therapies to treat patients that would be excluded from the eligibility requirements of CAR-T treatments based on fitness, logistics and access were supported. However, COMP stated that from a regulatory perspective, as there was a lack of demonstration of the product’s equivalence in terms of efficacy, safety and benefit/risk balance for the target patient population, they were not able to grant designation based on MCPC.
With improved early-stage myeloma outcomes for patients , novel treatments authorisations focussed on heavily pre-treated relapsed / refractory population. Single-arm clinical trial designs and changes to prior treatment algorithms make drawing comparisons across products in the late-line setting very challenging. Therefore, a creative, per product approach was required in recent years. Successful approaches have included;
Overall, consideration of how to best use all data sets available and robust statistical methods are the most appropriate approach.
Many factors impact EU access and reimbursement from company strategy to the type of data generated. Taking the recent authorisations in multiple myeloma it is not possible to draw any conclusions on whether maintained orphan designation later influenced reimbursement decisions. Data from the Medicines access portal generated by Myeloma Patients Europe shows a varied picture in late-line myeloma (Figure 2). For example, Germany reimburses all recent approvals, whereas France refused Tecvayli and Carvykti. Many products are available in various EU countries, but the speed of access from authorisation has not been assessed in this data, which is important for patients.
Figure 2. Outcome of reimbursement decisions in the EU on drugs authorised since 2020
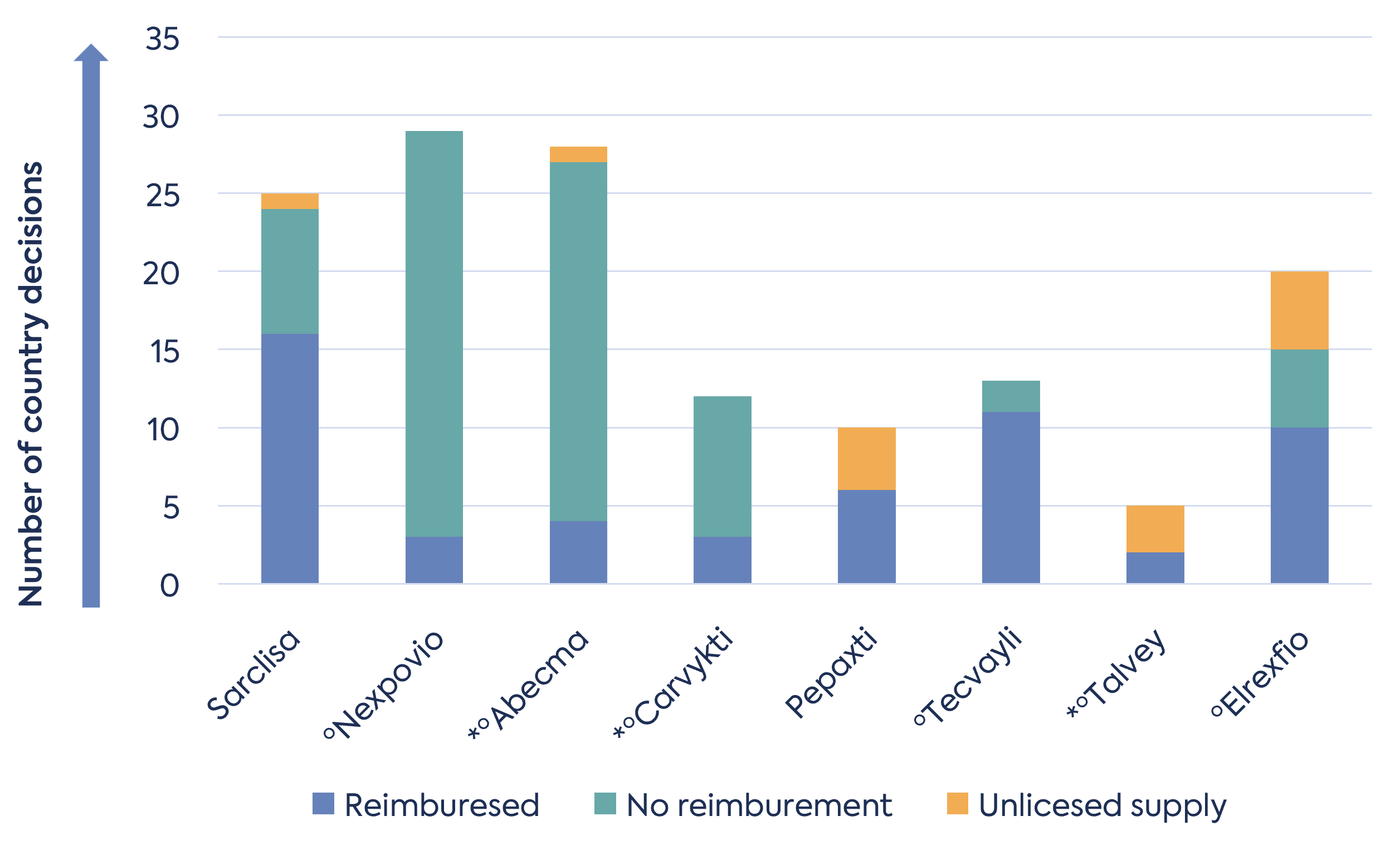
* indicates product maintained orphan designation at MAA
ºindicates the product received conditional MA (CMA).
Using multiple myeloma as a model, key learnings highlight the challenge of establishing significant benefit in competitive development.
Market insights from Fortune Business Insights Report ID: FBI100088
Source: https://www.fortunebusinessinsights.com/industry-reports/orphan-drugs-market-100088.

Published Mar 06, 2025

Published Feb 26, 2025

Published Feb 25, 2025

Published Feb 03, 2025

Published Feb 03, 2025

Published Jan 30, 2025

Published Jan 30, 2025

Published Jan 28, 2025

Published Jan 20, 2025