Antimicrobial Resistance (AMR): One Health Approach in the EU & UK
Published Nov 15, 2024
Published 15th November 2024

The rising threat of antimicrobial resistance (AMR) is coming closer to the fore of global political discussion. At the recent United Nations General Assembly, support for the multisectoral One Health approach was unanimous. Antimicrobial resistance (AMR) presents a significant unmet medical need, and regulators in the EU and the UK recognise the need for flexibility in their approach to requirements for medicine developers in this field. Early dialogue is important to maximise the opportunities to obtain financial and regulatory incentives for developers of novel antimicrobials.
A recent systematic analysis published by The Lancet predicts that 39 million people worldwide could die from antimicrobial resistant infection over the next 25 years. Delegates at the United Nations General Assembly High Level Meeting on Antimicrobial Resistance in September this year recognised the seriousness and global scale of the threat from antimicrobial resistance (AMR). They agreed on the need for urgent, coordinated action from world leaders to set targets for change, together with appropriate funding to support national action.
At the core of the political declaration approved by delegates at this meeting is a commitment to the multisectoral One Health approach to antimicrobial resistance (AMR). One Health emphasises the need to address the overlapping impact of human, animal and environmental factors in the development of resistance.
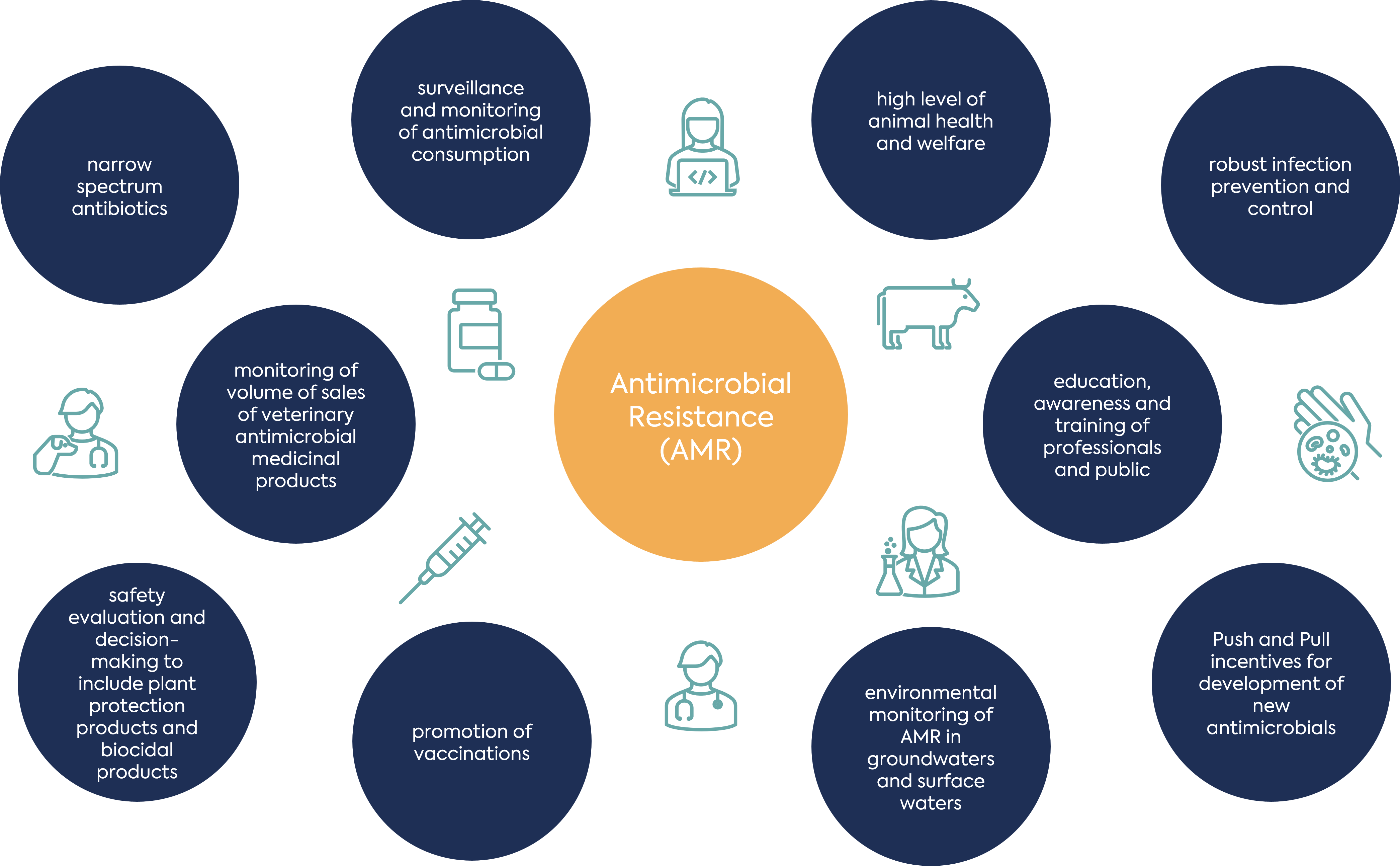
An understanding of the differing impacts of human, animal and environmental factors in different regions is important to feed into the design of effective and targeted One Health interventions.
A recent article in Nature Communications describes two relevant studies. The first was a study of extended spectrum beta-lactamase (ESBL) producing E. coli, conducted by the UK Health Security Agency. When isolates from human faeces, sewage, farm slurry, and retail foodstuffs were compared to human bloodstream infection (BSI) isolates, little overlap between non-human reservoirs and isolates from invasive human disease was found. This contrasted with a multivariable analysis of longitudinal human, animal and environmental sampling at individual households in the Drivers of Resistance in Uganda and Malawi (DRUM) study. This study found that human ESBL-producing E. coli colonisation was associated with the wet season and close animal interaction.
The European Commission welcomed the UN’s political declaration, highlighting antimicrobial resistance (AMR) as a top public health priority within the EU. The Commission has reiterated its commitment to One Health priorities, pointing to existing targets for the reduction of antibiotic consumption in humans, rules to limit antibiotics given to livestock, research and development investment for new antibiotics, and strengthened environmental monitoring to prevent antibiotic residues from contaminating soil and water.
The importance of public awareness of antimicrobial resistance (AMR) and the need for a change in individuals’ attitudes towards the use of antibiotics is also reflected in EU initiatives. These include a game-based educational campaign to raise awareness of antimicrobial resistance (AMR) specifically among young people.
Support for the One Health approach comes from a report published earlier this year by the European Centre for Disease Prevention and Control (ECDC), the European Food Safety Authority (EFSA), and the European Medicines Agency (EMA). It was shown that countries in the EU that have decreased their consumption of antibiotics in both animals and humans have seen a reduction in antibiotic-resistant bacteria.
Proposals to update EU pharmaceutical legislation contain specific measures to tackle antimicrobial resistance (AMR), addressing stewardship, prudent use and specific Environmental Risk Assessments for antimicrobials.
| Prudent use | Medical prescription throughout the EU (member states free to add further conditions e.g. special or restricted prescription, the imposition of limits to the amounts prescribed and/or mandatory diagnostic test use). Pack sizes to correspond to the usual posology and duration of treatment. Information on antimicrobial resistance (AMR) and appropriate use and disposal of antimicrobials to be added to the patient leaflet. |
| Stewardship plans | Information on risk mitigation measures to limit antimicrobial resistance (AMR) development, monitoring, and reporting to the competent authority information about resistance to the medicinal product. |
| Environmental risk assessments (ERAs) for antimicrobials | Requirement to address the risk of antimicrobial resistance (AMR) selection in the environment, due to the entire manufacturing, use and disposal lifecycle of such products. |
Such measures are vital, but new antimicrobials are also desperately needed. Traditional market incentives for the development of new antibiotics are limited, as confirmatory clinical trials can be large and expensive, and access to approved products needs to be restricted.
Both ‘push’ incentives, such as grants for early stage development, and ‘pull’ incentives to reward market access are important for antimicrobial developers. Recognising an urgent need, the European Commission considered several possible options to improve the impact of pull incentives. In April this year, MEPs adopted their position on the proposed legislation, with some modifications. Proposed incentives now include measures such as a milestone payment reward scheme, a subscription model procurement scheme and the use of Transferable Exclusivity Vouchers (TEVs). The legislation has not yet completed its first Council reading.
The EMA and the Heads of Medicines Agencies (HMA) recognise the importance of their role in supporting the development of innovative approaches to tackle AMR. Their draft joint EU network strategy to 2028 (EMAES 2028) offers support to the developers of new antimicrobials via the Emergency Task Force (ETF) and the Innovation Task Force (ITF). These groups allow enhanced access to regulators and scientific support to facilitate development and licencing.
The UK published a 20-year vision for antimicrobial resistance (AMR) in 2019 and will update its National Action Plan (NAP) every five years to support the delivery of these commitments. The second NAP (2024-2029), contains nine strategic outcomes organised under four themes.
| Theme 1: Reducing the need for, and unintentional exposure to, antimicrobials | • Infection prevention and control and infection management • Public engagement and education • Strengthened surveillance |
| Theme 2: Optimising the use of antimicrobials | • Antimicrobial stewardship and disposal • Raising awareness in the workforce in human and animal health and agriculture to improve the optimal use of antimicrobials |
| Theme 3: Investing in innovation, supply and access | • Innovation and influence within the life sciences sector • Using information to enable decisions to be based on robust surveillance, scientific research and data sets • Improving information available to identify where the burden of antimicrobial resistance (AMR) is greatest so that interventions can be targeted and health disparities and health inequalities can be reduced |
| Theme 4: Being a good global partner | • Antimicrobial resistance (AMR) diplomacy and sustained global engagement |
Within Theme 3 is the commitment to innovation and influence within the life sciences sector. This includes the implementation of a world first subscription model intended to de-link the price paid for antimicrobials from the volumes sold.
In the UK, the Academy of Medical Sciences’ FORUM, the Department of Health and Social Care (DHSC) and the National Institute for Health Research (NIHR) held a workshop in 2021 on lessons for antimicrobial resistance (AMR) that could be learned from the response to the COVID-19 pandemic. Among the themes that emerged from this discussion was the need for innovations in regulatory practice for the approval of therapeutics and vaccines.
The UK’s MHRA highlighted their collaborative efforts with researchers in the field of antimicrobial resistance (AMR) in the November 2023 MedRegs blog. These efforts include a focus on new technologies such as emerging microbiome research, bacteriophages, novel diagnostics, and new bacterial vaccines.
MHRA recognises that there is a lack of evidence to support the development of regulatory guidance for many of the novel approaches to tackling antimicrobial resistance (AMR) and that this could create delays in assessment. MHRA is actively attempting to engage with innovators as early as possible to identify products in development and prepare appropriate guidance for them before a submission for authorisation is made.
Over the next few months, we will be looking in more detail at some of the policy issues that will affect developers and Marketing Authorisation Holders, emerging scientific developments, and the regulatory environment in the field of antimicrobial resistance (AMR).
The failure rate for developers of medicinal products in this area is high, however, the rising political urgency around antimicrobial resistance (AMR) presents opportunities, not just for antibiotic development but also for vaccines, rapid diagnostic and antibiotic sensitivity tests, novel approaches to treatment of bacterial infections, such as bacteriophages, treatment for resistant antifungal infections and more.
There is recognition of the need for flexibility from regulators in the EU and the UK. DLRC Group has extensive experience of early and constructive interaction with regulators and strategic regulatory road mapping to support your development goals in this field. Contact us at hello@dlrcgroup.com to find out how we can assist you.

Published Nov 15, 2024

Published Nov 12, 2024

Published Oct 21, 2024

Published Oct 10, 2024
Published Oct 07, 2024
Published Sep 26, 2024

Published Sep 24, 2024
Published Sep 24, 2024

Published Sep 10, 2024