DLRC Achieves Cyber Essentials Plus Certification
Published Apr 11, 2025
Published 31st May 2024

Pharmacovigilance – the detection, monitoring, assessment and prevention of adverse effects of a drug – occurs during the entire lifecycle of a drug product. Safety information is collected and assessed at regular intervals, and the details are summarised in the form of a safety update report. Safety update reports are essential for protecting patient health, managing risks, and ensuring regulatory compliance. Regulatory authorities worldwide require pharmaceutical companies to submit safety update reports as part of the drug approval and post-marketing surveillance process. Compliance with these regulatory requirements is necessary to obtain and maintain marketing authorisation for any drug.
This article describes the differences and similarities in content, format, and submission between the development safety update report (DSUR) and the periodic safety update report (PSUR). Both are mandatory ICH regulatory documents that characterise a medicinal product’s safety profile and risk-benefit.
A DSUR contains safety information about a drug under clinical investigation and is updated regularly with new safety information from ongoing clinical trials.
A PSUR contains safety information about a drug after a marketing authorisation is granted. Real-world safety information is collected to determine whether there are new safety risks and/or if the risk-benefit ratio of the drug has changed, and actions to mitigate safety concerns and protect public health. (Note: a Periodic Benefit-Risk Evaluation Report [PBRER] is the same as a PSUR. The term used depends on the preference of the respective regulatory authority).
Preparation begins on the date the first interventional clinical trial is approved in any country, known as the developmental international birth date (DIBD). This is also the first day of the 1-year reporting period. The last day of each reporting period is known as the data lock point. The DSUR is updated annually until the clinical study report (CSR) of the final clinical trial before marketing authorisation is submitted. The latest DSUR update is either submitted as part of the marketing authorisation application, and/or the important risks reported in the DSUR are incorporated into a risk management plan (RMP), depending on the country/region.
Preparation begins on the date of first marketing authorisation/approval in any country, known as the international birth date (IBD). The PSUR is updated according to national/regional requirements and may not be required if the drug product is generic. The need for the submission of a PSUR and the frequency of report submission to regulatory authorities are subject to national or regional regulatory requirements and usually depend on such factors as approval dates, the length of time the product has been on the market, and the extent of knowledge of the benefit-risk profile of the product.
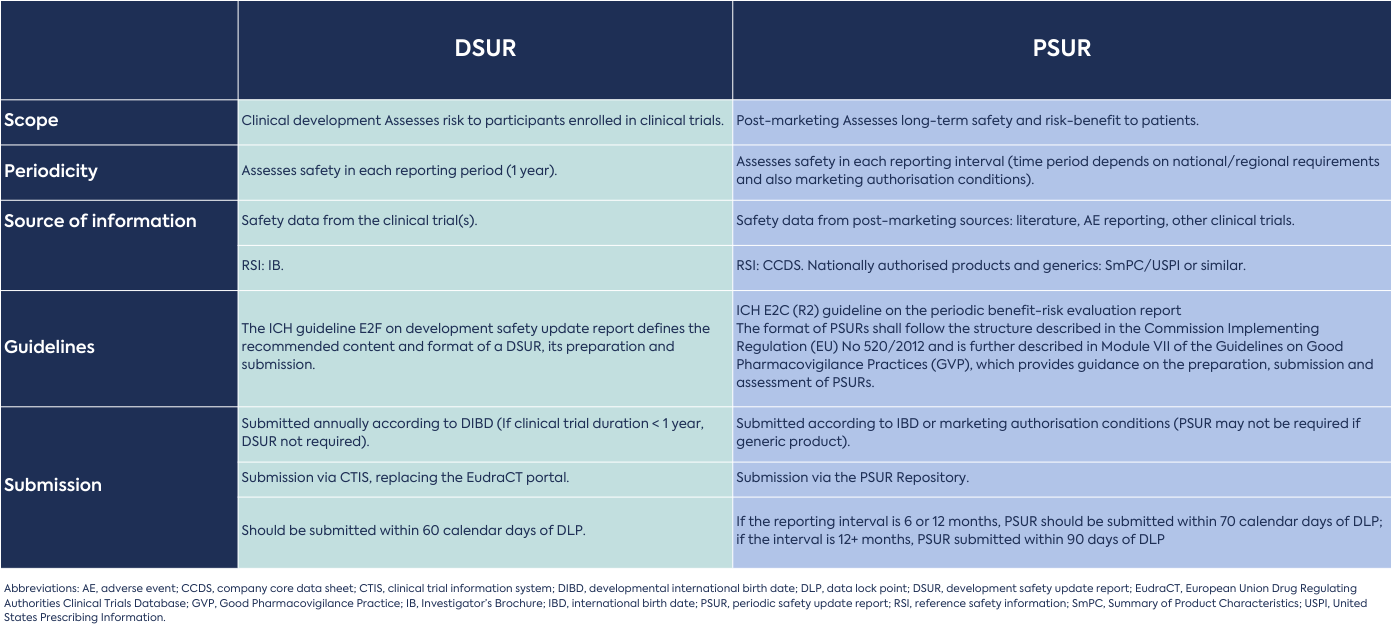
If clinical development of a drug continues after a marketing authorisation in any country/region (e.g., for Phase 4 therapeutic use trials), both a DSUR and PSUR may be submitted to regulatory authorities. In this case, the DSUR reporting period can be aligned to the IBD. However, the reporting period will still be one year in length.
There is considerable overlap between DSURs and PSURs in structure and content, and some repetition is expected. For example, information from marketing experience (reported in the PSUR) might be relevant to clinical development and, therefore, reported in the DSUR. Safety findings from clinical trials conducted using marketed drugs would be included in the DSUR. But, would also be pertinent to post-marketing safety and would be reported in the PSUR.
Both the DSUR and PSUR should be comprehensive and standalone. This is because they focus on a different subject matter and have differing periodicities and recipients. Due to the different scope and focus, the level of detail for common sections might differ between the DSUR and the PSUR. Evaluation of safety signals and benefit-risk assessment are more extensive in the PSUR. For products which require both a PSUR and a DSUR in the EU, the DSUR can be considered a subset of PSUR with a focus on the ongoing clinical trials.
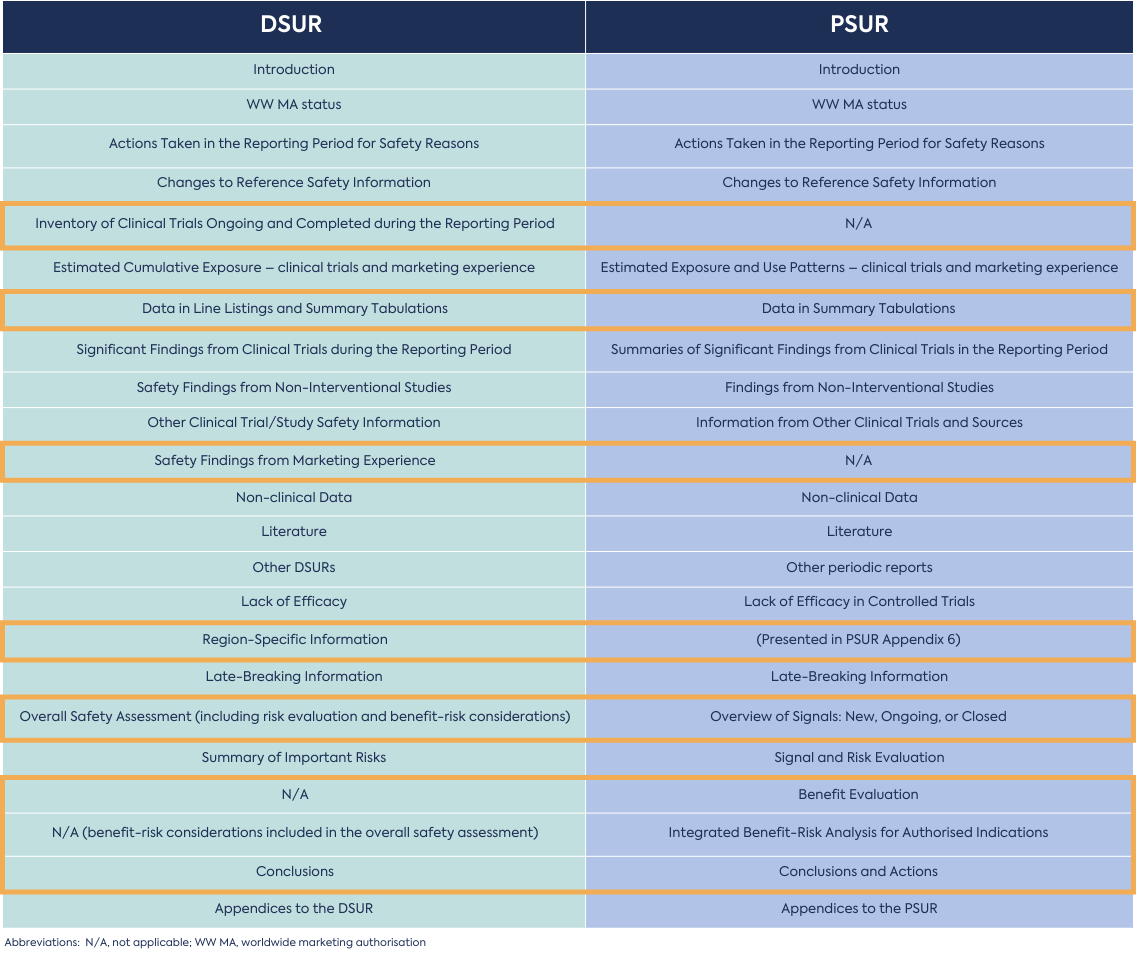
In simple terms, a DSUR refers to the clinical safety evaluation of a drug under clinical investigation, and a PSUR refers to the risk-benefit assessment of a drug after it has been authorised. Both documents have different periodicities and submission timings. Although there is an overlap in the structure and content of both documents, the scope and focus differ, with the DSUR being more focused on the evolving safety profile and the PSUR being focused on benefit-risk analysis.
DLRC’s team of highly experienced medical writers are available to support you with the preparation of pharmacovigilance documents, including, but not limited to, DSURs and PSURs. For any related queries, contact DLRC at hello@dlrcgroup.com or use the link below.

Published Apr 11, 2025

Published Mar 27, 2025

Published Mar 25, 2025

Published Mar 06, 2025
Published Feb 26, 2025

Published Feb 25, 2025

Published Feb 03, 2025

Published Feb 03, 2025

Published Jan 30, 2025